Researchers do it in the lab, at room temperature.
This article is best suited for Year 7 Physics students who are learning about pressure and forces as well as Year 4, 8 and 10 Chemistry students learning about atomic structure and Year 8 Earth and Space students learning about the Earth’s resources.
Word Count: 631

Australian-led research has taken a leaf out of Superman’s comic book and created diamonds at room temperature for the first time.
Diamond is a substance made of pure carbon, crushed into a crystal structure that is both transparent and incredibly hard. Though it usually takes billions of years to form, this new study produced diamonds in the lab in mere minutes.
The key was to apply pressures of 100 gigapascals (GPa) – equivalent to 640 African elephants balanced on the point of a ballet shoe.
The results are published in the journal Small.
Jodie Bradby, co-author from the Australian National University (ANU) in Canberra, muses that Superman might have used the same high-pressure trick when he used his super strength to crush coal into diamond – without the need for his heat vision.
“Natural diamonds are usually formed over billions of years, about 150 kilometres deep in the Earth where there are high pressures and temperatures above 1000 degrees Celsius,” she explains.
The minimum pressure required for natural diamond formation is around 15-20 GPa. Previous research has used high-pressure, high-temperature methods to simulate this environment in the lab and thus rapidly form diamonds, but Bradby and colleagues took a different approach.
By applying pressure to a material called “glassy carbon” in a very specific way, they circumvented the need for extreme temperatures.
“The twist in the story is how we apply the pressure,” Bradby says. “As well as very high pressures, we allow the carbon to also experience something called ‘shear’ – which is like a twisting or sliding force. We think this allows the carbon atoms to move into place.”
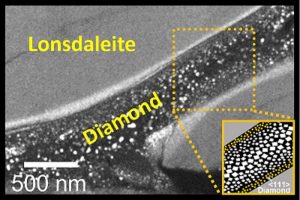
The team produced two types of diamond: the familiar bling found on engagement rings, and the rare Lonsdaleite, which is naturally found at the site of meteorite impacts.
Bradby’s group at ANU made the diamonds, while lead author Dougal McCulloch’s team inspected the experimental samples at the RMIT Microscopy and Microanalysis Facility in Melbourne. By using advanced electron microscopy techniques, they created snapshots of how the two different diamonds formed.
“Our pictures showed that the regular diamonds only form in the middle of these Lonsdaleite veins under this new method,” McCulloch says. “Seeing these little ‘rivers’ of Lonsdaleite and regular diamond for the first time was just amazing and really helps us understand how they might form.”
Lonsdaleite has a different crystal structure to regular diamond and is predicted to be 58% harder, and so may have useful applications.
“Lonsdaleite has the potential to be used for cutting through ultra-solid materials on mining sites,” says Bradby. “Creating more of this rare but super useful diamond is the long-term aim of this work.”
The samples used were quite small, approximately 0.05mm square, but McCulloch is positive that they can scale up.
“Given we discovered that shear plays an important role in the formation process, there are apparatus designed to increase the shear – known as shear cells,” he says.
“We are now trying to go further and see if we can lower the pressure further to 5 GPa in a larger shear cell, which will allow larger quantities to be made.”
Although Superman created diamonds from coal, the team will be choosing their source material more carefully – a polycrystalline form of graphite, which makes the largest and best quality diamonds for commercial applications.
In fact, McCulloch has a few pro tips for Superman.
“Coal is very disordered and contains a lot of impurities, so without heat would likely produce poor quality diamonds,” he explains.
Instead, he suggests, Superman could switch up his material and attempt to introduce shear to the process.
“Our work would suggest that if he started with graphite, he could indeed make good quality diamonds at room temperature,” McCulloch concludes.
If only McCulloch had been a scientific advisor for the Superman franchise.
Years: 4, 7, 8, 10
Login or Sign up for FREE to download a copy of the full teacher resource