One approach to the control of carbon emissions is carbon capture.
This excellent explainer article discusses the different forms of renewable energy and the impact they have on both individuals lives and the world. The easy-to-understand terminology makes this ideal for Chemistry students who are learning about CO2 emissions and technological advances that help reduce levels in the atmosphere. It is also an accessible piece for Year 6 Earth and Space and Year 8 Physics students.
Word Count: 738

Carbon capture and storage (CCS)
When a coal, oil or gas plant burns fuel to create electricity, a major by-product is the greenhouse gas carbon dioxide (CO2).
One approach to keeping carbon emissions under control is the use of carbon capture and storage (CCS) technologies that use underground rocks as “storage tanks”. But how do these technologies work?
When fossil fuels are burnt they produce a range of different gases including oxygen, nitrogen and CO2.
CCS focuses on selectively pulling this CO2 out of the gas mixture and preparing it for underground storage. Three main approaches have been developed to do this: pre-combustion, post-combustion and oxyfuel combustion.
Pre-combustion
As the name says, a pre-combustion setup focuses on capturing CO2 before the fuel is burnt.
First, an air separator strips oxygen from the atmosphere, producing an almost pure stream of oxygen gas. This is then fed into a unit known as the gasifier, which bakes the coal at around 700°C, releasing a mixture of gases including hydrogen, carbon monoxide, CO2 and steam. Collectively this is known as syngas.
By adding water to this syngas in a shift reactor, it is converted into hydrogen and CO2. Separating these two gases produces a stream of hydrogen, which is burnt off, and CO2, which is dehydrated to remove any leftover water and compressed to concentrate the gas into a liquid form for transport and storage.
To maximise the efficiency of the process, the heat produced by burning the hydrogen is redirected to convert water to steam and so produce more electricity using conventional steam turbines.
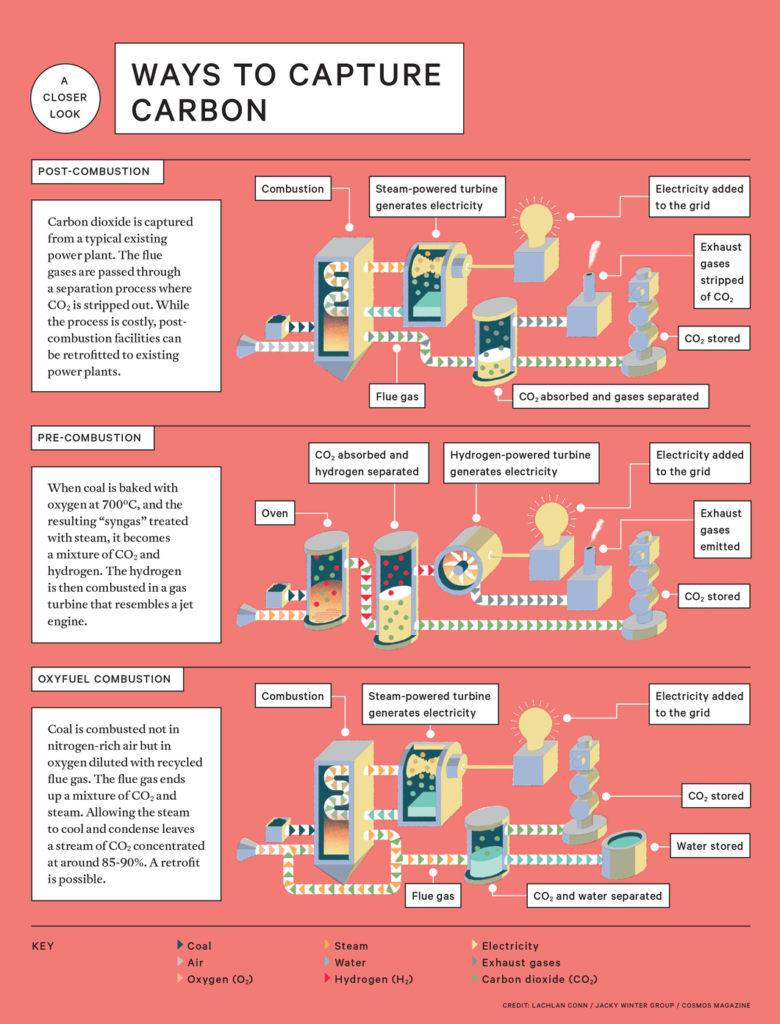
Post-combustion
Post-combustion is another technique used to capture CO2. It has the advantage of being able to be retrofitted to existing power plants.
Fuel is injected into a boiler with air and burnt in the same way you would typically find at a coal, oil, or gas-fired power plant.
Also: Renewable Energy
The heat produced inside this boiler is used to convert water to steam that in turn powers a set of turbines to produce energy.
The by-product of this burn is a mixture of nitrogen, CO2 and water collectively termed flue gas.
A wide variety of filtration systems can pluck the CO2 from this mixture. Some examples currently used or being investigated are ultra-porous crystals, ammonia and limestone membranes that can selectively bind and release CO2, and even populations of algae or cyanobacteria which feed on the gas to survive.
This filtration pulls the CO2 from the flue gas, which can then be dehydrated and compressed ready for transport and storage.
Oxyfuel combustion
Oxyfuel combustion systems burn coal using flue gas and pure oxygen, produced with an air separation unit. From this reaction comes heat, which is used to convert water to steam, and a mixture of flue gas and water.
This mixture can be used to regulate the temperature of the boiler before being passed through a CO2 purification unit that first removes other pollutants including sulfur and nitrogen.
It then compresses the CO2 and separates it from other non-reactive gases including oxygen and nitrogen to produce a stream of water that has a very high concentration of CO2.
Storage
Once the CO2 has been captured from the energy production process it is ready to be stored.
After transportation by trucks or pipeline, the liquid gas is pumped into porous rock formations that can be kilometres below the surface.
At these depths, the temperature and pressure keep the gas in its liquid form where it is trapped within the geological layer.
Depleted oil fields are often used as storage tanks because a large amount of geological data is readily available, produced during the prospecting process.
The most important part of selecting a storage site is the presence of an impermeable rock layer above the porous rock known as “cap rock”, which prevents the liquid gas from escaping.
Log in or Sign up for FREE to download a copy of the full teacher resource