How does the freezer work? — Leon, aged 4.
To answer this, we need to understand solids, liquids and gases as well as heat energy and physical changes.
Learn how a freezer stays cold in this resource for Year 1-8 Chemistry and Physics students learning about states of matter and heat energy.
Word Count: 739

Hi Leon,
That’s a great question! But freezers are a bit tricky to explain, so we’ll need to talk about a few other things first.
Everything you can touch and feel (like air, water, rocks and mice) is made of tiny balls called atoms. When atoms join up into small groups moving around together, they are called molecules. Atoms and molecules are too small to see without very powerful microscopes.
Solids, liquids and gases
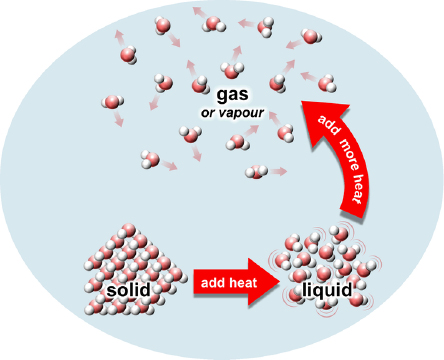
Most things come in three phases: solid, liquid or gas. Think of ice, water and steam. If a gas is not too hot, we can also call it vapour. (There are other phases too, but let’s ignore them for today.)
In solids (like ice), atoms or molecules are tightly stuck together and can barely move. They are usually lined up in neat rows called crystals. In liquids (like water) atoms or molecules are loosely stuck close together, but can move around. In a gas (like steam), atoms or molecules are far apart and free to float away from each other.
Most gases, including air, are made of small molecules. Some gases (like the helium inside floating party balloons) are made of single atoms moving around on their own.
If I heat up a solid, the atoms or molecules start to bounce a little bit, but they still stay stuck in their neat rows. Now, if I add an extra burst of heat, the solid turns into liquid. This means the atoms and molecules bounce around so hard they start to move around, breaking up those neat rows. Although the atoms can now flow around, they still stay very close together. This is what’s happening if you put an ice block in a bowl and watch it slowly melt into water.
To turn a liquid into a gas (or vapour), the atoms and molecules must break away completely from their neighbours. This takes another extra burst of heat to give the atoms and molecules a kick to rip them away from their sticky neighbours and float away. (Scientists call this extra burst of heat latent heat.)
This is what happens when you put water into a kettle, turn on the heat, and watch the steam floating out of the spout.
These atoms or molecules carry that extra burst of heat away with them when they float away. This is why your face feels cooler if the wind turns your sweat into vapour and floats away from your face.
OK. Now let’s try it backwards. If you take enough heat out of a vapour (like steam), it will turn back into a liquid (like water). Whenever this happens, the vapour brings the extra burst of heat back into the liquid.
Now, finally, I can explain how your freezer works.
How the freezer works… at last!
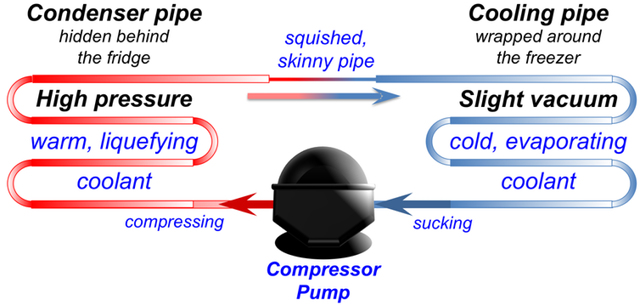
Hidden inside the walls of your freezer is a curly metal tube called a cooling pipe. It is full of a special liquid that evaporates easily.
The cooling pipe is connected to a pump that sucks in vapour from the cooling pipe. The sucking makes more liquid turn to vapour, and when that happens it takes some heat out of the freezer. Just like sweat floating away cools your face down, this vapour floating away makes the inside of the freezer cool down.
Next, the pump takes vapour from the cooling pipe and squeezes it into another curly pipe on the outside of the back of the fridge. When the pump squeezes the vapour, it pushes the molecules closer together so they start to stick together and turn into a liquid again.
When the gas turns back into a liquid, it gives off the latent heat energy it took from the freezer. So the pipe on the back of the fridge gets warm, and the heat escapes into the air in your kitchen.
In other words, the pump moves heat from inside your freezer and lets it go into your kitchen, making the freezer colder and your kitchen warmer. If you feel the back and sides of your fridge, they should feel a bit warm. That’s the heat that used to be inside your freezer!
After releasing its heat energy, the liquid leaks through a little skinny pipe back into the cooling pipe where it started. Then the sucking from the pump turns it into gas again, and the whole cycle repeats over and over. And that’s what keeps your freezer cold.
![]()
Answered by Stephen G Bosi, Senior Lecturer in Physics, University of New England
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Login or Sign up for FREE to download a copy of the full teacher resource
Years: 1, 2, 3, 5, 6, 8
Topics:
Chemical Sciences – Atoms, Solids/Liquids/Gases, Particle Models
Physical Sciences – Energy
Additional – Careers, Technology.
Concepts (South Australia):
Chemical Sciences – Properties of Matter, Change of Matter
Physical Sciences – Energy
 Curious Kids is a series by The Conversation that gives children the chance to have their questions about the world answered by experts. If you have a question you’d like an expert to answer, send it to [email protected].
Curious Kids is a series by The Conversation that gives children the chance to have their questions about the world answered by experts. If you have a question you’d like an expert to answer, send it to [email protected].
We won’t be able to answer every question, but we’ll do our very best.
When sending in questions to Curious Kids, make sure you include the asker’s first name, age and town or city. You can:
- email [email protected]
- tweet us @ConversationUK with #curiouskids
- DM us on Instagram @theconversationdotcom