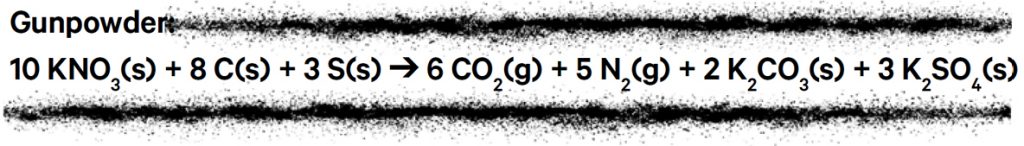Ever wondered about what’s behind the big bangs and bright sparks of New Year’s Eve? Nathan Kilah ignites the discussion.
Seeing the relevance of chemical reactions on students everyday life can be hard for them, however the importance of fireworks at many celebrations and festivals across many different cultures is a way to show how an understanding of science can lead to explosively good fun.
This article from Cosmos magazine issue 85 and resource could be used with students from Year 6-12 to deliver the Chemical Sciences curriculum.
Word Count: approx. 1315
The back of Launceston-based pyrotechnician Tony Sumner’s shirt reads: “Pyrotechnician. If you see me running, try to keep up.” It’s an old joke, but it makes sense. Fireworks are dangerous things. Seemingly harmless sparklers caused over 500 emergency room visits in the US in 2018. At times, even the professionals get things wrong. In San Diego in 2012, a 7000-firework, 17-minute Independence Day display was fired in a ferocious – and blinding – 30 seconds.
Fireworks are integral to many cultural celebrations: what would Chinese New Year, New Year’s Eve or the Fourth of July be without them? I’ve found that demonstrations combined with professional firework displays are a great way to engage the community with chemistry. To paraphrase the American physicist Richard Feynman, the beauty isn’t just at the larger scale, but also in the smaller dimensions, and how they work together.
Fireworks are broadly defined as explosive devices that emit a combination of coloured flames, sparks, and noises, and can be made to climb high into the sky before exploding. It’s fundamental chemistry and physics coming together in the blink of an eye in an awe-inspiring display. There are few all-encompassing sensory experiences like watching a fireworks display: the visceral thud of the lifting charge, the anticipation as the faint glow of the lit fuse rises like a tiny flare, the smell of heavy smoke and sulfur oxides in the air, and the revelry as colour and sparkle explode across the sky.
A Tale as Old as Time
The breakthrough for the development of fireworks was the creation of gunpowder. Ninth-century Chinese alchemists were searching for elixirs to extend life, and stumbled across mixtures of honey, potassium nitrate and sulfur that erupted into flames when heated in a fire.
Over time the honey was replaced by charcoal, and a proto-scientific method of trial and error gave rise to gunpowder mixtures very closely resembling those used today. The general formulation for gunpowder (also known as black powder) is 75% potassium nitrate (KNO3, an oxidant), 15% charcoal (a carbon-rich fuel) and 10% sulfur (S, a flux that lowers the ignition temperature of the mixture).
Alchemists searching for elixirs to extend life stumbled across mixtures of honey, potassium nitrate and sulfur that erupted into flames.
These ingredients need to be present in the correct quantities; the size of the particles and how well they’re dispersed will have a direct impact on how rapidly they burn, deflagrate (a subsonic, but rapid, oxidation) or explode.
The ingredients are simple, but the chemical reactions taking place are very complex. Below is a simplified, balanced equation for the combustion of gunpowder.
The solid potassium nitrate, charcoal and sulfur combine to produce the gases carbon dioxide (CO2) and nitrogen (N2), which rapidly expand when the powder explodes, while solid products potassium carbonate (K2CO3 ) and potassium sulfate (K2SO4 ) are suspended in the air as thick smoke. Many other by-products are produced, including oxides of nitrogen and sulfur, carbon monoxide (CO), metal salts and particles. Fireworks rightly don’t have a particularly clean reputation: many of these compounds are environmental pollutants.
Let’s Get Physical
The colours we see in fireworks are a result of the quantum physics of atoms. Atoms have a nucleus containing protons and neutrons surrounded by electrons. These electrons are present at specific, known energy levels. A useful analogy is to think of the electrons as balls sitting on a staircase. The electrons can rise or fall between the steps, but the distances between the steps remains the same.
In fireworks, metal atoms are excited by the combustion of gunpowder, raising their electrons to higher energy levels. The electrons don’t stay at the higher energy levels, but quickly return to their lower energy state by releasing light. The light released is characteristic of the element (and is known as its emission spectrum), as all atoms have the same “staircase” of quantised electron energies.
So, adding certain metal salts to the fireworks will dependably give specific colours: barium salts – BaCl2, BaCO3, Ba(NO3)2, and Ba(ClO3)2 – for green, copper salts – CuCl, CuCO3, and CuO – for blue, sodium salts – NaNO3, Na2 (oxalate), and Na3AlF6 – for orange, strontium salts – SrCO3, Sr(NO3)2, and SrSO4 – for red, and mixtures for other colours.
Burning pieces of solid metal also contribute to the colour effects seen in fireworks, sparklers, and lance-works (“firework” words and shapes, usually on a ground-based frame). The hot burning pieces of metal are ejected while reacting with oxidants, and with oxygen in the air. Metals used include aluminium (Al), magnesium (Mg) and titanium (Ti), which burn with intense white light, while iron (Fe) gives the sparks an orange colour.
Whistle Works
Colours aren’t the only effects: noise is an important feature of fireworks displays. Thuds and bangs are sonic booms (exploding gases from gunpowder expanding faster than the speed of sound), while crackling effects are made by mixing a magnesium-aluminium (Mg-Al) alloy with a metal oxide – historically, lead (II, IV) oxide (Pb3O4), but increasingly bismuth oxide (Bi2O3) as an environmentally conscious choice. This mixture is divided into granules that burn rapidly to give the crackling noise.
Whistles are made from highly volatile compositions, for example mixtures of oxidants – such as potassium perchlorate (KClO4) – and fuels like sodium benzoate, sodium salicylate, or highly unstable potassium picrate. This mixture is packed within a partially filled tube. As the compound burns, rapidly expanding gases create standing waves within the tube, resonating like an organ pipe or a whistle. As the composition is burnt away, the length of the pipe changes, shifting the pitch.
Packing for Fun
The streaks of light we see from aerial fireworks come from small pellets of gunpowder, metals and metal salts, and binders, which are poetically named “stars”. These are formed by cutting a block of the mixed materials into small cubes (think a party platter of cheddar cheese), pressing them into round pellets, or even rolling them into spheres in tumbling machinery.
The rolling technique allows for the manufacture of layered “gobstoppers” of different compositions, which display changing colours and sparkles as they burn. The stars are typically packed into spherical shells or cylinders ready for ignition.
Modern ignition systems make use of “e-matches” – small match-shaped mixtures of oxidants like potassium perchlorate (KCIO4) or potassium nitrate (KNO3) with metal or metal-hydride fuels, connected to wires.
An electrical charge is passed through the wire, igniting the e-match and creating hot sparks that are directed at the firework fuse. E-matches allow the modern pyrotechnician to impeccably time computer-controlled sequences, which may be synchronised with music, or co-ordinated across multiple firing positions.
Up, Up and Away
The majority of fireworks at a modern professional display are shells fired from mortar tubes. The fuse is ignited, setting off a gunpowder lifting charge that hurls the spherical shell high into the air. The burning fuse reaches the interior of the shell and ignites the bursting charge, which explodes the shell and ignites the stars that have been carefully packed inside.
The arrangement of the stars around the bursting charge allows for the creation of shapes in 360°. These can be floral-named spherical arrangements like chrysanthemums and peonies (incorporating coloured stars with or without sparkling trails), hearts, smiling faces, letters and more.
More complex and expensive cylindrical fireworks allow for the layering of effects, such as an initial colour, followed by a different colour higher, then a larger sparkling charge. The possibilities and combinations are endless and limited only by the creativity of the pyrotechnician and your bank balance – it’s genuinely a case of more bang for your buck.
The fuse is ignited, setting off a gunpowder charge that hurls the spherical shell high into the air.
The typical, up-in-the-sky, public–display fireworks described here are just the tip of the pyrotechnic nose cone: this collection of chemicals can be used to create rockets, crackers, fountains, roman candles, cakes, wheels, spinners… and many more. So, next time you see some fireworks, take a moment between your ooohs and aaahs to consider the physics and chemistry that put them there. And if you see the pyrotechnician running away – well, yes; it’s probably a good idea to follow them, and fast!
This article was written by Dr Nathan Kilah, senior lecturer in Chemistry at the University of Tasmania for Cosmos Magazine Issue 85.
Cosmos magazine is Australia’s only dedicated print science publication. Subscribe here to get your quarterly fill of the best Science of Everything, from the chemistry of fireworks to cutting-edge Australian innovation.
Login or Sign up for FREE to download the educational resources