New system can work with low-grade feedstock.
This interesting article details an invention to use biodiesels through an innovative chemical process. It is suitable for Year 7, 8, 9, and 10 Chemisty, Physics and Earth and Science students learning about renewable energy, energy transfer and chemical reactions.
Word Count: 362

Australian and British researchers have developed a new, low-cost way to make biodiesel from used cooking oil and agricultural waste.
And with a little further development, they say, it could be tailored to produce jet fuel from agricultural and forestry waste, old rubber tyres and even algae.
The key is an ultra-efficient catalyst that can process low-grade feedstock containing up to 50% contaminants, reducing the need for energy-intensive and expensive initial cleaning. Conventional catalyst technologies depend on feedstocks with only 1-2% contamination.
The work – by a team from RMIT University, the University of Western Australia, University College London, University of Manchester, University of Plymouth, Aston University, Durham University and University of Leeds – is described in a paper in Nature Catalysis.
To make the new catalyst, they fabricated a micron-sized ceramic sponge (just 1/100th the width of a human hair) that is highly porous and contains specialised active components.
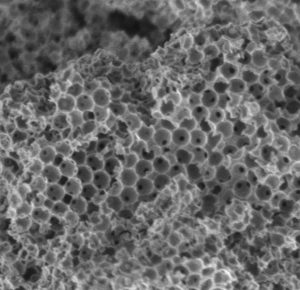
Molecules initially enter the sponge through large pores, where they undergo a first chemical reaction, then pass into smaller pores where they undergo a second reaction.
It’s the first time, the authors write, that a multi-functional catalyst has been developed that can perform several chemical reactions in sequence within a single catalyst particle.
“Catalysts have previously been developed that can perform multiple simultaneous reactions, but these approaches offer little control over the chemistry and tend to be inefficient and unpredictable,” says co-lead investigator Karen Wilson, from RMIT.
“Our bio-inspired approach looks to nature’s catalysts – enzymes – to develop a powerful and precise way of performing multiple reactions in a set sequence.
“It’s like having a nanoscale production line for chemical reactions, all housed in one, tiny and super-efficient catalyst particle.”
The sponge-like catalysts are cheap to manufacture because they use no precious metals. All that’s needed is a large container and some gentle heating and stirring.
The next stage is to scale up the fabrication from grams to kilograms and use 3D printing to accelerate commercialisation.
“We’re also hoping to expand the range of chemical reactions to include light and electrical activation for cutting-edge technologies like artificial photosynthesis and fuel cells,” says RMIT’s Adam Lee.
This article was curated for Cosmos. Read the original article.
Years: 7, 8, 9, 10
Login or Sign up for FREE to download a copy of the full teacher resource